FDA Approves Pfizer-BioNTech COVID-19 Vaccine
Pfizer-BioNTech vaccine, now called Comirnaty, is approved for individuals 16 and over
September 13, 2021
The Food and Drug Administration (FDA) approved Pfizer-BioNTech COVID-19 vaccine for individuals 16 and older on Aug. 23, 2021 under the FDA’s priority review process.
Priority review is for “the evaluation of applications for drugs that, if approved, would be significant improvements in the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions when compared to standard applications.”
These drugs will be reviewed within six months rather than the 10-month deadline for standard review.
Drugs that receive priority review still have to go through the same length of clinical trials and meet the same quality standards for scientific and medical evidence.
The vaccine is still available for individuals ages 12 to 15 under Emergency Use Authorization (EUA). The EUA also applies to third doses for immunocompromised individuals.
The FDA approved COVID-19 vaccine will now be marketed as Comirnaty.
In order to get Comirnaty or any vaccine FDA approved, the manufacturer must submit a biologics license application (BLA), which includes even more extensive data and information than what manufacturers have to include in an EUA application. Manufacturers have to include information such as
- Preclinical and clinical data and information
- Details of the manufacturing process
- Vaccine testing results to ensure vaccine quality
- Inspections of the sites where the vaccine is made
“Our scientific and medical experts conducted an incredibly thorough and thoughtful evaluation of this vaccine. We evaluated scientific data and information included in hundreds of thousands of pages, conducted our own analyses of Comirnaty’s safety and effectiveness, and performed a detailed assessment of the manufacturing processes, including inspections of the manufacturing facilities,” said Peter Marks, M.D., Ph.D., director of FDA’s Center for Biologics Evaluation and Research.
“We have not lost sight that the COVID-19 public health crisis continues in the U.S. and that the public is counting on safe and effective vaccines. The public and medical community can be confident that although we approved this vaccine expeditiously, it was fully in keeping with our existing high standards for vaccines in the U.S.,” Marks continued.
Though the Comirnaty COVID-19 vaccine is now FDA approved. The FDA is still requiring more research into the risks of myocarditis, an inflammation of the heart muscle that reduces its ability to pump blood, and pericarditis, the usually mild “swelling and irritation of the thin, saclike tissue surrounding your heart.” A pregnancy registry study is also being conducted to analyze outcomes in pregnancy and infants after mothers have been vaccinated with Comirnaty during pregnancy.
Georgia Southern University does not offer the Cormirnaty COVID-19 vaccine because of its ultra-cold storage requirements but Walgreens, CVS and the Department of Public Health in Chatham County offer the Comirnaty vaccine.
Visit the Department of Public Health website or vaccines.gov website to learn where you can receive the Comirnaty vaccine. Students can also call 1-800-232-0233 or 888-720-7489 for deaf and hard of hearing individuals to help them find a vaccine location.
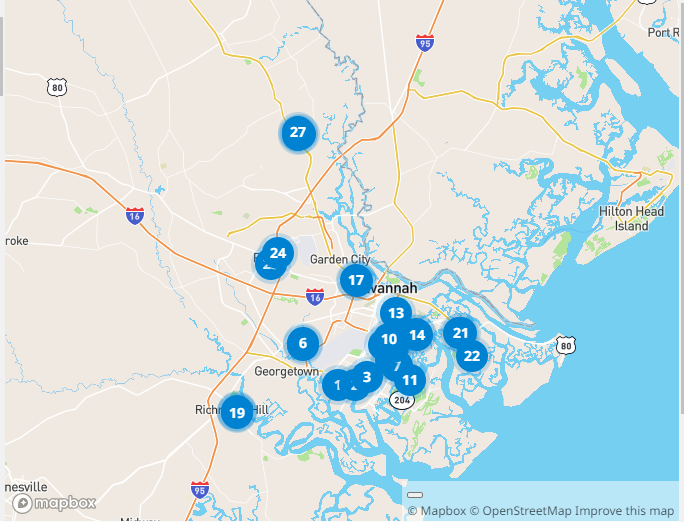
See the image at the top of this article to view all the locations within 25 miles of the Armstrong campus that offer the Comirnaty vaccine.